![This video on acids and bases shows you how to calculate the pH, pOH, [H+], [OH-] of acid and base solutions. Acids and bases can be measured by their stren... Identifying Lewis Acids and Lewis Bases 001 - YouTube](https://i.ytimg.com/vi/5JUA3Uqpw60/default.jpg)


![Add some acid. Add some conjugate base. What's the pH? This is the old-school way. You can also use the Henderson-Hasselbalch Equation. In this example I use... Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and ...](https://i.ytimg.com/vi/gKju83Cz3kk/default.jpg)






![This video on acids and bases shows you how to calculate the pH, pOH, [H+], [OH-] of acid and base solutions. Acids and bases can be measured by their stren... Identifying Lewis Acids and Lewis Bases 001 - YouTube](https://i.ytimg.com/vi/5JUA3Uqpw60/default.jpg)


![Add some acid. Add some conjugate base. What's the pH? This is the old-school way. You can also use the Henderson-Hasselbalch Equation. In this example I use... Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and ...](https://i.ytimg.com/vi/gKju83Cz3kk/default.jpg)






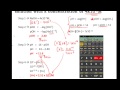
Question: What Is The Conjugate Base Of NH3? This problem has been solved! See the answer. What is the conjugate base of NH3? Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. NH4+ is the conjugate acid to the base NH3, because NH3 gained a hydrogen ion to form NH4+.The conjugate base of an acid is formed when the acid donates a proton. + conjugate acid of a strong base (NaOH). CO3 2-conjugate base of a weak acid (HCO 3-). Therefore the solution will be basic. KI K+ conjugate acid of a strong base (KOH). I-conjugate base of a strong acid (HI). Therefore the solution will be neutral. NH4Cl NH4 + conjugate acid of a weak base (NH 3). Cl-conjugate base of a strong acid (HCl). The conjugate acid of ammonia is the ammonium ion, NH_4^+. The conjugate acid of any species, is the original species PLUS a proton, H^+. Both mass and charge are conserved. So add a H^+ unit to NH_3, and I gets NH_4^+, ammonium ion. Are both mass and charge conserved here? By the same procedure, if I remove H^+ from any species, I get the conjugate base. When NH3 acts as a base, it will donate its lone pair to a proton H+ and form its conjugate acid NH4+ whereas when NH3 acts as an acid, it can give out H+ ion and forms a conjugate base as NH2-. Reactions are given below: (Acting as a Lewis Base) NH3 + H+ ——-> NH4+. (Acting as a Lewis Acid) NH3 ——–> NH2- + H+. well NH3 is a base that reacts with H2O to get NH4 + OH- NH3+ H2O-->NH4+ + OH- A conjugate base is the species formed when a Bronsted- Lowry base accepts a proton. NH4+ is the conjugate acid of NH3. Nitrate, or NO3-, is the conjugate base of HNO3. The reaction resulting in the conjugate base of HNO3 is HNO3 + H2O → H3O+ + NO3-. In most cases, the acid molecule that remains after losing a hydrogen ion is an acid's conjugate base. NH3 + H+ = NH4+ and NH3 is the conjugate base, NH4+ the conjugate acid. However, in aprotic solvents (e.g. tetrahydrofuran) NH3 can be an acid. NH3 + B → NH2- + HB. Now, NH3 is the conjugate acid, NH2- is the conjugate base. For this to occur, ‘B’ has to be a pretty strong base - e.g., n-butyl lithium will do. The conjugate base of bicarbonate, HCO 3- is carbonate, CO3 2-.. HCO3- is a conjugate acid, H 2 CO 3 3.34 Draw the conjugate base for each of the following acids: (a) OH (Ь) (c) NH3 (d) H,0 ZT le) OH (7) (g) NH. COMPANY About Chegg
[index] [1416] [1258] [9273] [316] [472] [9830] [7628] [4913] [4597] [828]
This video on acids and bases shows you how to calculate the pH, pOH, [H+], [OH-] of acid and base solutions. Acids and bases can be measured by their stren... This chemistry video tutorial explains how to calculate the pH of a buffer solution using the henderson hasselbalch equation. It explains the concept, compo... A step-by-step explanation of how to draw the NO3- Lewis Structure (Nitrate Ion). Get more chemistry help at www.Breslyn.org.For the NO3- Lewis structure,... Add some acid. Add some conjugate base. What's the pH? This is the old-school way. You can also use the Henderson-Hasselbalch Equation. In this example I use... This chemistry video tutorial explains how to calculate the percent ionization of a weak acid and base given Ka or Kb. This video provides the percent disso... In this video we will describe the equation CH3OH + H2O and write what happens when CH3OH is mixed with water.When CH3OH is mixed with H2O (water) there isn’... In this video we will describe the equation NaOH + H2O and write what happens when NaOH is dissolved in water.When NaOH is dissolved in H2O (water) it will d... Identify the Lewis acid and Lewis base in the following reaction:SO3 + H2O ⇌ H2SO4 This chemistry video tutorial shows you how to identify an ionic compound as acidic, basic, or a neutral salt. You need to know the 6 common strong acids su... This chemistry video tutorial provides a basic overview / introduction to titrations. It shows you how to calculate the unknown concentration of an acid sol...
Copyright © 2024 top.playrealmoneygametop.xyz